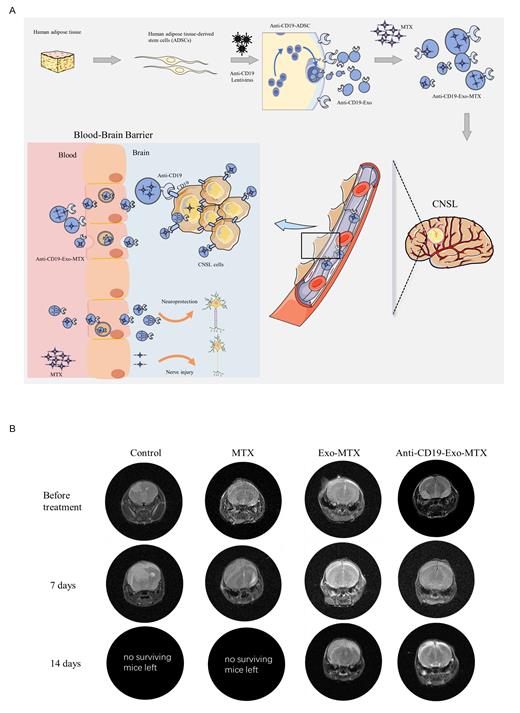
High-dose methotrexate (HDMTX) serves as the cornerstone of central nervous system lymphoma (CNSL) treatment, but its efficacy is limited due to low Blood-Brain Barrier (BBB) penetration (2% to 20%) and adverse effects like myelosuppression and hepatotoxicity. Thus, this study focuses on exosome-based drug delivery to enhance BBB permeability, reducing methotrexate (MTX) dosage while targeting CNSL specifically (Figure 1A).
Considering the recipient's compatibility and reduced risk of adverse reactions or rejection, exosomes derived from human adipose tissue-derived stem cells (ADSCs) were selected as drug delivery vehicles. ADSCs-Exo underwent purification through ultracentrifugation and were characterized using nanoparticle tracking analysis (NTA) and transmission electron microscope (TEM).
CD19 is considered an excellent target for immunotherapy in DLBCL. To confer targeting capabilities, ADSCs were infected with a lentiviral vector encoding the anti-CD19 prior to the purification of exosomes. The anti-CD19 (patent number: ZL201811653085.8) was strongly expressed in ADSCs, integrated into ADSCs-derived exosomes, and localized on the external exosomal surface, as evidenced by western blots, colloidal gold immunoelectron microscopy, and flow cytometry.
Based on the properties of MTX, we employed co-incubation to load MTX into anti-CD19-modified exosomes. The resulting exosomes, loaded with MTX, were then quantified for the encapsulated MTX using High Performance Liquid Chromatography.
The anti-CD19-modified exosome-loaded methotrexate (anti-CD19-Exo-MTX) interacted with cerebrovascular endothelial cells and astrocytes of the BBB, leading to endocytosis and facilitating MTX transportation across the barrier. Additionally, anti-CD19-Exo-MTX crossed the BBB model without compromising its structure, as assessed by dextran's luminescent signal assay. Compared to drug-free formulations, anti-CD19-Exo-MTX exhibited enhanced BBB permeability, thereby improving its capability to inhibit CNSL cell proliferation.
Liquid chromatography-tandem mass spectrometry identified and quantified proteins in exosomes, revealing significant enrichment of neuron projection and axon development-associated proteins. Subsequent neurite outgrowth assays, Nissl staining, and modified neurological severity scores confirmed the beneficial effects of ADSCs-Exo in mitigating MTX-induced neuron damage.
To evaluate the efficacy of anti-CD19-Exo-MTX in vivo, intracranial orthotopic CNSL models in mice were established. Magnetic resonance imaging (MRI) analysis revealed a reduced disease burden in the anti-CD19-Exo-MTX group compared to both the MTX and Exo-MTX groups (Figure 1B), along with prolonged overall survival. Cerebrospinal fluid (CSF) drug concentration analysis demonstrated enhanced stability and longer-lasting drug levels for anti-CD19-Exo-MTX in the CSF, while showing no toxicity to vital organs, as evidenced by the absence of significant histological damage in the heart, liver, spleen, lung, and kidney tissues.
To assess biodistribution and targeting of anti-CD19-Exo-MTX, exosomes were labeled with VivoTrack Dil (Fluorescence). Intracranial fluorescence signal was observed for anti-CD19-Exo-MTX, while Exo-MTX exhibited systemic distribution. Two hours post-injection, organs removal revealed weaker fluorescence signals in the liver and spleen for anti-CD19-Exo-MTX, confirming its specific targeting of MTX to CNSL in vivo.
In conclusion, we developed anti-CD19-Exo-MTX, an exosome-based drug delivery platform, demonstrating enhanced BBB permeability and specific targeting for CNSL. This promising approach offers potential for effective CNSL treatment with reduced adverse effects.
Disclosures
No relevant conflicts of interest to declare.